Subtopic 1.3: Lipids
Lipid molecules are mainly hydrophobic molecules i.e. found in areas away from water molecules, but also present smaller hydrophilic parts that are important for its biological function. The major roles of lipid molecules are to serve as storage of biological energy (Example: triacylglycerols) and provide the building blocks for biological membranes (Example: phospholipids and cholesterol). Although there are other types of lipids, in this topic we will discuss the structure and function of these main groups of lipids.
Triacylglycerols
Triacylglycerols are composed of fatty acids and glycerol.
Triacylglycerols
Triacylglycerols are composed of fatty acids and glycerol.
- Glycerol is a simple three-carbon molecule with hydroxyl groups at each carbon.
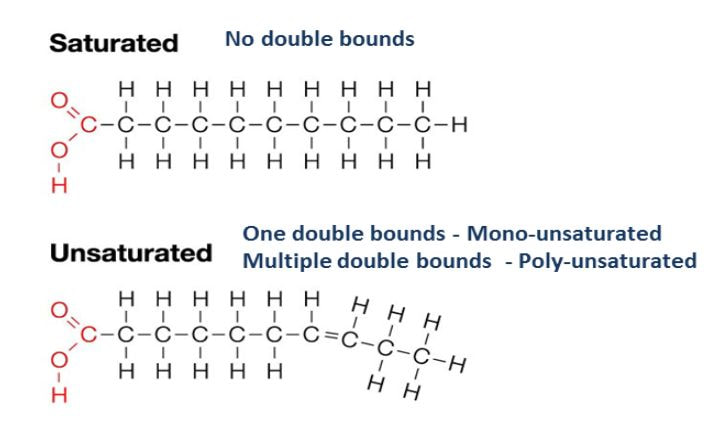
- Fatty acids are chains of carbon molecules with a carboxylic acid (COOH) in the first carbon and a CH3 (methyl) group at the end of the chain.
|
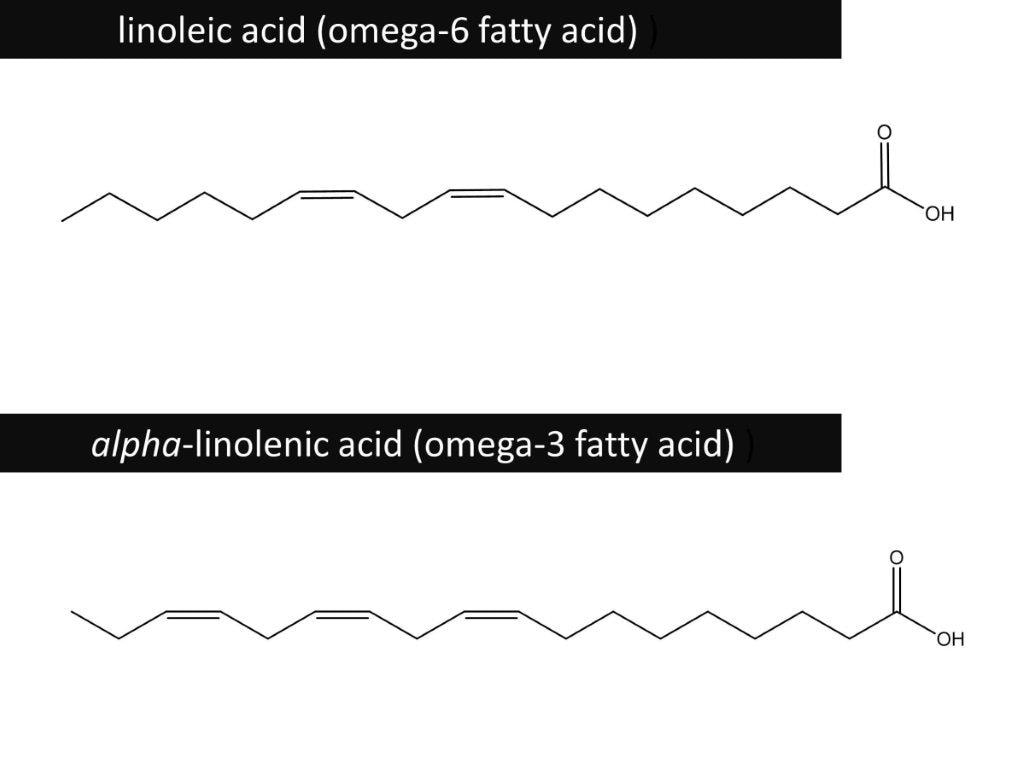
Fatty acids can be saturated or unsaturated.
|
|
Essential Fatty Acids
|
Phospholipids
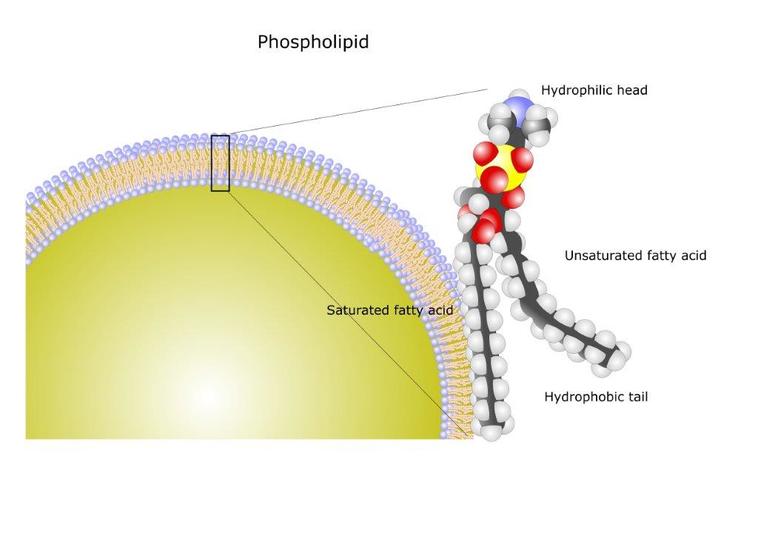
Phospholipids are the major component of cell membranes. They form lipid bilayers because of their amphiphilic characteristic.
The structure of the phospholipid molecule generally consists of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group (PO4−3) attached to the third glycerol carbon. This head group is usually charged, creating a part of the lipid that is hydrophilic, and wants to be near water, a quality that is essential for the formation of biological membranes and many lipid functions.
Phospholipids are the major component of cell membranes. They form lipid bilayers because of their amphiphilic characteristic.
The structure of the phospholipid molecule generally consists of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group (PO4−3) attached to the third glycerol carbon. This head group is usually charged, creating a part of the lipid that is hydrophilic, and wants to be near water, a quality that is essential for the formation of biological membranes and many lipid functions.
|
Steroids
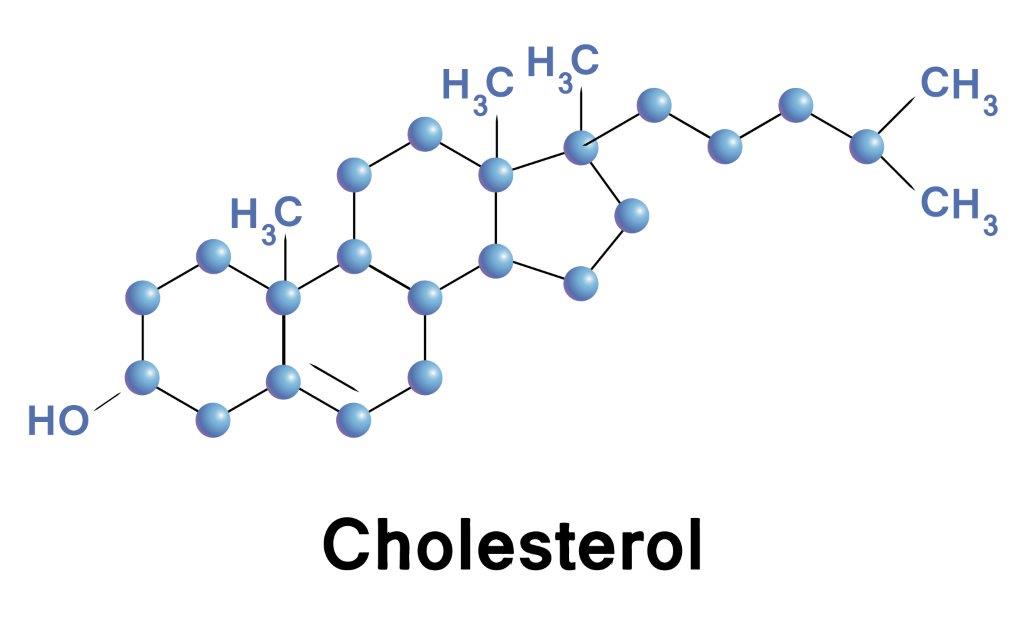
Steroids are lipids that have four rings made of carbon atoms—three rings have six sides and one has five sides—with a six-carbon ring tail. Examples: bile salts, cholesterol, the sexual hormones estrogen, progesterone and testosterone, corticosteroids and pro-vitamin D. Cholesterol is an important molecule found only in eukaryotic organisms with a variety of functions. Cholesterol is also a component of biological membranes and its main function is to control the fluidity of membranes. Cholesterol does not like to be exposed to water environments, preferring to be shielded by other hydrophobic molecules such as lipids or hydrophobic parts of proteins Cholesterol also serves as the primary source for the production of steroid hormones, bile salts, and vitamin D. |