Hazard Identification
|
The goal of hazard identification in toxicology is to identify or develop information suggesting or confirming that a chemical (or, for example, a consumer product) poses or does not pose a potential hazard to humans. During earlier years of toxicology, this process relied primarily on human epidemiology data and on various types of animal testing data, supplemented in more recent years with the development of in vitro methods such as those focused on assessing the potential for mutations and DNA damage. The future of hazard identification is promising and toxicologists now have various types of in vitro methods to explore for hazard identification, along with the emergence of "chip" approaches. |
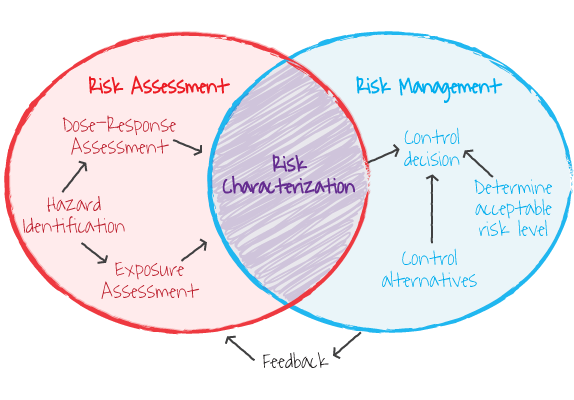
Figure 1. Hazard identification is the first component of risk assessment
(Image Source: ORAU, ©) |
These emerging methods are based, in part, on (Quantitative) Structure Activity, or (Q)SAR methods. Q(SAR) methods, such as computer models, help toxicologists and others to consider closely related chemicals as a group, or chemical category, rather than as individual chemicals. Not every chemical needs to be tested for every toxicity endpoint, and the data for chemicals and endpoints that have been tested are used to estimate the corresponding properties for other chemicals and endpoints of interest. Data from a chemical category must be judged as adequate to support at least a "screening-level" hazard identification.
One approach involves using endpoint information for one chemical to predict the same endpoint for another chemical that is considered "similar" in some way (such as having structural similarity and similar properties and/or activities).
Read-Across
Another approach for hazard identification used since about 2000 is read-across. Read-across can be qualitative or quantitative:
- In qualitative read-across, the presence (or absence) of a property/activity such as a particular type of toxic effect for the chemical of interest is inferred from the presence (or absence) of the same property/activity for one or more other chemicals. This qualitative approach provides a "yes/no" answer.
- Quantitative read-across uses information for one or more chemicals to estimate what the chemical of interest will be like. Thus, quantitative read-across can be used to obtain a quantitative value for an endpoint, such as a dose-response relationship.
Adverse Outcome Pathways (AOPs)
An emerging approach to hazard identification is the use of Adverse Outcome Pathways (AOPs). AOPs reflect the move in toxicity testing from high-dose studies in laboratory animals to in vitro methods that evaluate changes in normal cellular signaling pathways using human-relevant cells or tissues. The AOP concept has emerged as a framework for connecting high throughput toxicity testing (HTT, or high throughput toxicity screening, HTS) and other results.
AOP Learning Channel
The Human Toxicology Project Consortium provides a collection of informational videos about AOPs for your further exploration. These videos are available on the AOP Learning Channel.
Other Computer Models
Another emerging term is (quantitative) in vitro to in vivo extrapolation, or (Q)IVIVE, used together with what are being called Integrated Testing Strategies and Integrated Approaches to Testing and Assessment (IATA).
Toxicology Testing in the 21st Century - A New Strategy
AOP Learning Channel
The Human Toxicology Project Consortium provides a collection of informational videos about AOPs for your further exploration. These videos are available on the AOP Learning Channel.
Other Computer Models
Another emerging term is (quantitative) in vitro to in vivo extrapolation, or (Q)IVIVE, used together with what are being called Integrated Testing Strategies and Integrated Approaches to Testing and Assessment (IATA).
Toxicology Testing in the 21st Century - A New Strategy
|
The High Throughput Screening (HTS) Initiative is part of the new toxicology testing strategy developed from the 2004 National Toxicology Program (NTP) Vision and Roadmap for the 21st Century.
Traditional toxicological testing is based largely on the use of laboratory animals. However, this approach suffers from low throughput, high cost, and difficulties inherent to inter-species extrapolation – making it of limited use in evaluating the very large number of chemicals with inadequate toxicological data. NTP recognized that the dramatic technological advances in molecular biology and computer science offered an opportunity to use in vitro biochemical- and cell-based assays and non-rodent animal models for toxicological testing. These assays allow for much higher throughput at a much reduced cost. In some assays, many thousands of chemicals can be tested simultaneously in days. The goal is to move toxicology from a predominantly observational science at the level of disease-specific models to a predominantly predictive science focused upon broad inclusion of target-specific, mechanism-based, biological observations. The High Throughput Screening program represents a new paradigm in toxicological testing. The HTS program approach to toxicological testing screens for mechanistic targets active within cellular pathways considered critical to adverse health effects such as carcinogenicity, reproductive and developmental toxicity, genotoxicity, neurotoxicity, and immunotoxicity in humans. |
Figure 2. National Toxicology Program vision and roadmap
(Image Source: National Toxicology Program) |
Goals of the HTS Program
National Toxicology Program. (2016, March 21). Tox 21. U.S. Department of Health and Human Services. Retrieved from http://ntp.niehs.nih.gov/results/tox21/index.html
- To prioritize substances for further in-depth toxicological evaluation.
- To identify mechanisms of action for further investigation (for example, disease-associated pathways).
- To develop predictive models for in vivo biological response (predictive toxicology).
National Toxicology Program. (2016, March 21). Tox 21. U.S. Department of Health and Human Services. Retrieved from http://ntp.niehs.nih.gov/results/tox21/index.html
As described in the Testing for, and Assessing Toxicity section, the EPA is developing "Virtual Tissue Models" such as the Virtual Embryo (v-Embryo™). These types of advanced computer models are being designed to be capable of simulating how chemicals may affect human development and will help reduce dependence on animal study data. They will also provide faster ways of developing chemical risk assessments.
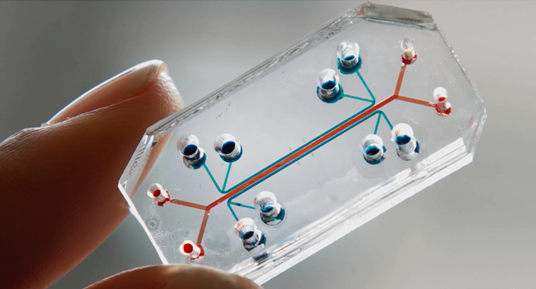
Finally, also noted in the Testing for and Assessing Toxicity section, emerging in the toxicologist's tool box are "chip" models (for example, an "organ on a chip"). One example is the "Lung-on-a-chip" that "…offers a new in vitro approach to drug screening by mimicking the complicated mechanical and biochemical behaviors of a human lung."
Figure 3. Lung-on-a-chip used to mimic pulmonary edema
(Image Source: The Wyss Institute for Biologically Inspired Engineering)
(Image Source: The Wyss Institute for Biologically Inspired Engineering)
Knowledge Check (Solutions on next page)
1) Modern approaches to hazard identification are based in part on:
a) Extensive animal testing for toxicity
b) Computer models like (Q)SAR
c) Risk assessment strategies
2) Adverse Outcome Pathways (AOPs) are methods of hazard identification that:
a) Evaluate changes in normal cellular signaling pathways using human-relevant cells or tissues
b) Identify adverse outcomes in test subjects administered increasing doses of potential toxicants
c) Measure the intensity, frequency, and duration of human exposures to agents
d) Evaluate the health effects under various conditions of human exposure
3) Can quantitative read-across be used to determine the value of an endpoint, such as dose-response relationship?
a) Yes
b) No