Testing for and Assessing Toxicity
Alternatives to animal testing have emerged in recent years.
Since about 1990, numerous attempts have been made around the world to reduce the use of and replace laboratory animals in toxicology and other studies. These efforts have involved finding alternatives to animal testing and incorporating the "3Rs" concept (Replace, Reduce and Refine), which means using test methods that:
Regulatory authorities, companies, and others have endorsed the principle of the 3Rs, and alternative testing methods have been and are being developed. An international group that has played a key role is the International Cooperation on Alternative Test Methods (ICATM). Established in 2009, ICATM includes representatives of organizations from various countries as seen below.
The International Cooperation on Alternative Test Methods (ICATM) includes representatives from:
Finding Information about Alternatives to Animal Testing
Many countries including the United States, Canada, and the European Union member states, require that a comprehensive search for possible alternatives be completed before some or all research involving animals is begun. Because numerous Web resources are now available to provide guidance and other information on in vitro and other alternatives to animal testing, completing such searches and keeping current with information associated with alternatives to animal testing is much easier than it used to be.
Alternatives to animal testing have emerged in recent years.
Since about 1990, numerous attempts have been made around the world to reduce the use of and replace laboratory animals in toxicology and other studies. These efforts have involved finding alternatives to animal testing and incorporating the "3Rs" concept (Replace, Reduce and Refine), which means using test methods that:
- Replace the use of animals with other types of studies and approaches.
- Reduce the number of animals in studies.
- Refine the procedures to make studies less painful or stressful to the animals.
Regulatory authorities, companies, and others have endorsed the principle of the 3Rs, and alternative testing methods have been and are being developed. An international group that has played a key role is the International Cooperation on Alternative Test Methods (ICATM). Established in 2009, ICATM includes representatives of organizations from various countries as seen below.
The International Cooperation on Alternative Test Methods (ICATM) includes representatives from:
- Korean Centre for the Validation of Alternative Methods (KoCVAM)
- U.S. Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM)
- U.S. National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM)
- Japanese Center for the Validation of Alternative Methods (JaCVAM)
- Health Canada
- European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM)
- Other organizations working on alternative methods, such as from Brazil and China.
Finding Information about Alternatives to Animal Testing
Many countries including the United States, Canada, and the European Union member states, require that a comprehensive search for possible alternatives be completed before some or all research involving animals is begun. Because numerous Web resources are now available to provide guidance and other information on in vitro and other alternatives to animal testing, completing such searches and keeping current with information associated with alternatives to animal testing is much easier than it used to be.
ALTBIB, from NLM
|
The NLM ALTBIB ("Resources for Alternatives to the Use of Live Vertebrates in Biomedical Research and Testing") portal is a comprehensive starting point for finding information related to alternatives to animal testing. ALTBIB is available at https://www.nlm.nih.gov/enviro/altbib.html.
It provides access to PubMed®/MEDLINE® citations relevant to alternatives to use of live vertebrates in biomedical research and testing. ALTBIB's topics and subtopics are aligned with current approaches. For example, information is provided on in silico, in vitro, and improved (refined) animal testing methods and on testing strategies that incorporate these methods and other approaches. |
Figure 1. NLM ALTBIB homepage
|
ALTBIB also provides access to news and additional resources, including information on the status of the evaluation and acceptance of alternative methods. Main categories include:
Links to Specific Resources (Sources Providing Animal Alternatives News, Key Organizations Providing Resources, and the Regulatory Acceptance of Specific Alternative Methods and Milestones in Non-animal Toxicity Testing)
- Animal Alternatives News
- Additional Resources
- Evaluation/Acceptance of Test Methods
Links to Specific Resources (Sources Providing Animal Alternatives News, Key Organizations Providing Resources, and the Regulatory Acceptance of Specific Alternative Methods and Milestones in Non-animal Toxicity Testing)
Animal Tests
NOTE: This information is provided for historical and other reasons, especially since animal testing is still being done in some cases, and because toxicologists, risk assessors, and others are faced with interpreting the results of new and old studies that used animals.
NOTE: This information is provided for historical and other reasons, especially since animal testing is still being done in some cases, and because toxicologists, risk assessors, and others are faced with interpreting the results of new and old studies that used animals.
|
Animal tests for toxicity have been conducted prior to and in parallel with human clinical investigations as part of the non-clinical laboratory tests of pharmaceuticals. For pesticides and industrial chemicals, human testing is rarely conducted. Years ago, results from animal tests were often the only way to effectively predict toxicity in humans.
Animal tests were developed and used because:
|
Figure 2. Rats have traditionally been used in toxicity studies using animals
(Image Source: iStock Photos, ©) |
Standardized Animal Toxicity Tests
Animal methods to evaluate toxicity have been developed for a wide variety of toxic effects. Some procedures for routine safety testing have been standardized. Standardized animal toxicity tests have been highly effective in detecting toxicity that may occur in humans. As noted above, concern for animal welfare has resulted in tests that use humane procedures and only as many animals as are needed for statistical reliability.
To be standardized, a test procedure must have scientific acceptance as the most meaningful assay for the toxic effect. Toxicity testing can be very specific for a particular effect, such as dermal irritation, or it may be general, such as testing for unknown chronic effects.
Standardized tests have been developed for the following effects:
Species Selection
Species selection varies with the toxicity test to be performed. There is no single species of animal that can be used for all toxicity tests. Different species may be needed to assess different types of toxicity. The published literature (such as via PubMed) and online databases (such as TOXNET) should be searched for information from non-animal and animal studies, as well as for possible best approaches, most applicable species, and strains and gender of a species. Here are two examples:
Animal methods to evaluate toxicity have been developed for a wide variety of toxic effects. Some procedures for routine safety testing have been standardized. Standardized animal toxicity tests have been highly effective in detecting toxicity that may occur in humans. As noted above, concern for animal welfare has resulted in tests that use humane procedures and only as many animals as are needed for statistical reliability.
To be standardized, a test procedure must have scientific acceptance as the most meaningful assay for the toxic effect. Toxicity testing can be very specific for a particular effect, such as dermal irritation, or it may be general, such as testing for unknown chronic effects.
Standardized tests have been developed for the following effects:
- Acute Toxicity
- Subchronic Toxicity
- Chronic Toxicity
- Carcinogenicity
- Reproductive Toxicity
- Developmental Toxicity
- Dermal Toxicity
- Ocular Toxicity
- Neurotoxicity
- Genetic Toxicity
Species Selection
Species selection varies with the toxicity test to be performed. There is no single species of animal that can be used for all toxicity tests. Different species may be needed to assess different types of toxicity. The published literature (such as via PubMed) and online databases (such as TOXNET) should be searched for information from non-animal and animal studies, as well as for possible best approaches, most applicable species, and strains and gender of a species. Here are two examples:
- It would have been invaluable years ago for toxicologists and risk assessors to have known that carcinogenic effects in male rats are considered irrelevant for humans if the α(2u)-globulin protein is involved because humans lack that protein. See another example
- Many physiological, pharmacological, and toxicological findings related to organic anion and cation transport and transporters in rodents and rabbits do not apply to humans. Learn more
- For example, use of dogs and non-human primates is now restricted to special cases or banned by some organizations, even though they represent the species that may respond the closest to humans in terms of chemical and other exposures (however, note the examples above).
|
The toxicologist attempts to design an experiment to duplicate the potential exposure of humans as closely as possible. For example:
|
Figure 3. Rodents have commonly been used in animal testing
(Image Source: iStock Photos, ©) |
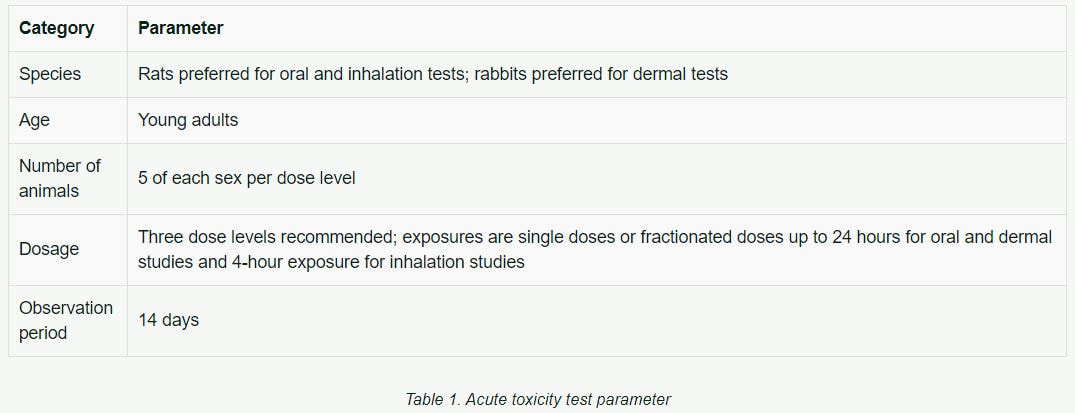
Acute Toxicity
Historically, acute toxicity tests were the first tests conducted. They provide data on the relative toxicity likely to arise from a single or brief exposure, or sometimes multiple doses over a brief period of time. Standardized tests are available for oral, dermal, and inhalation exposures, and many regulatory agencies still require the use of all or some of these tests. Table 1 lists basic parameters historically used in acute toxicity testing.
Subchronic Toxicity
Subchronic toxicity tests are employed to determine toxicity likely to arise from repeated exposures of several weeks to several months. Standardized tests are available for oral, dermal, and inhalation exposures. Detailed information is obtained during and after the study, ranging from body weight, food and water consumption measurements, effects on eyes and behavior, composition of blood, and microscopic examination of selected tissues and organs.
Table 2 lists basic parameters previously used in subchronic toxicity testing.
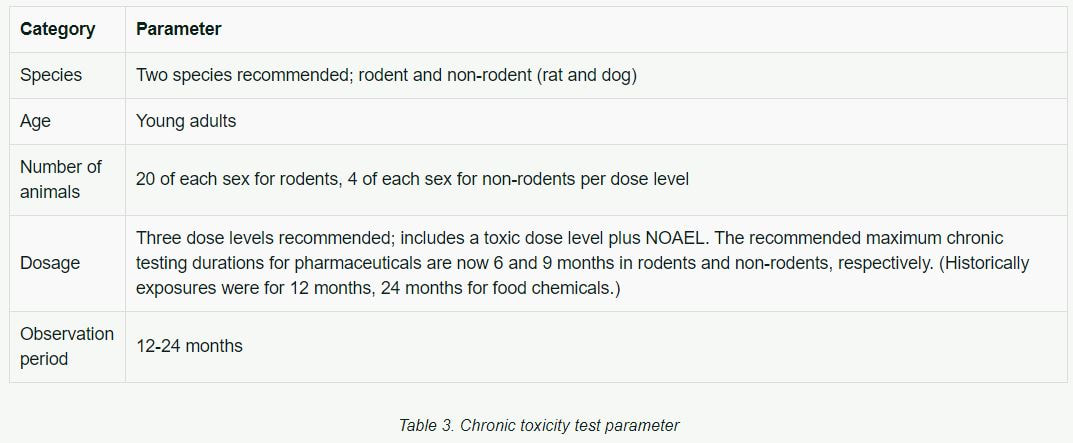
Chronic Toxicity
Chronic toxicity tests determine toxicity from exposure for a substantial portion of a subject's life. They are similar to the subchronic tests except that they extend over a longer period of time and involve larger groups of animals.
Table 3 includes basic parameters previously used in chronic toxicity testing.
Carcinogenicity
Carcinogenicity tests are similar to chronic toxicity tests. However, they extend over a longer period of time and require larger groups of animals in order to assess the potential for cancer.
Table 4 lists basic parameters used in the past in carcinogenicity testing.
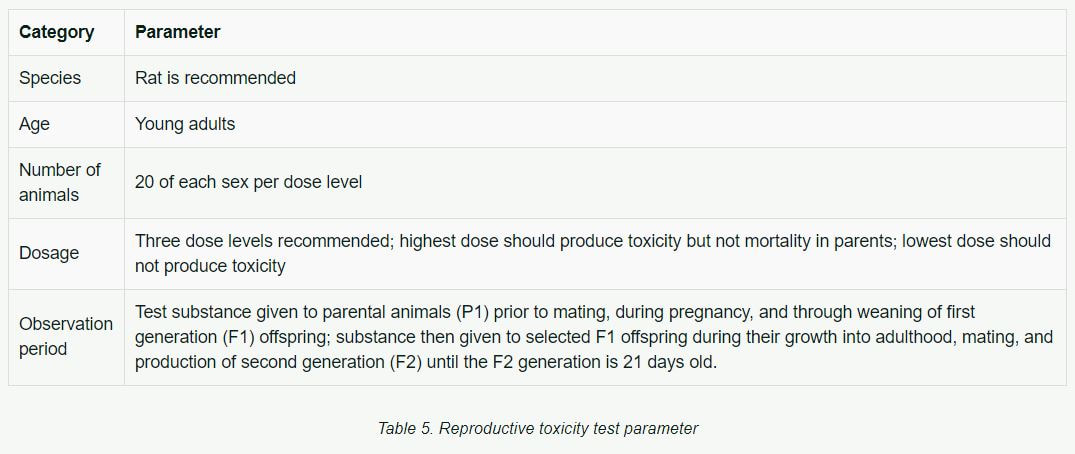
Reproductive Toxicity
Reproductive toxicity testing is intended to determine the effects of substances on gonadal function, conception, birth, and the growth and development of offspring. The oral route of administration is preferable.
Table 5 lists basic parameters historically used in reproductive toxicity testing.
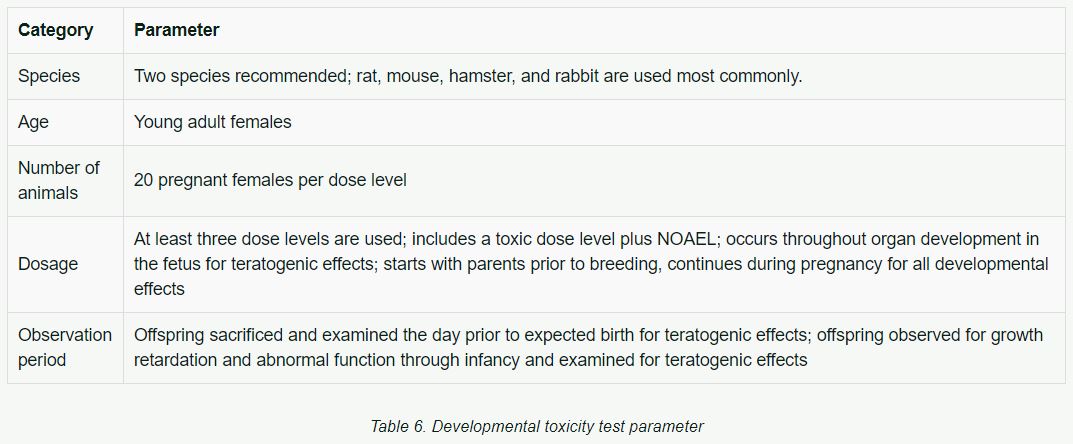
Developmental Toxicity
Developmental toxicity testing detects the potential for substances to produce embryotoxicity and birth defects.
Table 6 lists basic parameters previously used in developmental toxicity tests.
Dermal Toxicity
Dermal toxicity tests determine the potential for an agent to cause irritation and inflammation of the skin. Those reactions may be a result of direct damage to the skin cells by a substance or an indirect response due to sensitization from prior exposure. In vitro approaches to dermal toxicity testing are being developed, in part because this type of testing has received so much publicity.
Table 7 lists basic parameters historically used in dermal toxicity testing.
Ocular Toxicity
Ocular toxicity was at one time determined by applying a test substance for 1 second to the eyes of 6 test animals, usually rabbits. The eyes were then carefully examined for 72 hours, using a magnifying instrument to detect minor effects. An ocular reaction can occur on the cornea, conjunctiva, or iris. It may be simple irritation that is reversible and quickly disappears.
This eye irritation test was commonly known as the "Draize Test." This test has received much attention, such as the development of a "low volume" variation and in vitro approaches.
Neurotoxicity
A battery of standardized neurotoxicity tests were developed to supplement the delayed neurotoxicity test in domestic chickens (hens). The hen assay determines delayed neurotoxicity resulting from exposure to anticholinergic substances, such as certain pesticides. The hens are protected from immediate neurological effects of the test substance and observed for 21 days for delayed neurotoxicity.
Table 8 lists measurements included in other neurotoxicity tests.
Genetic Toxicity
Genetic toxicity is determined using a wide range of test species including whole animals and plants (for example, rodents, insects, and corn), microorganisms, and mammalian cells. A large variety of tests have been developed to measure gene mutations, chromosome changes, and DNA activity.
Table 9 lists parameters used for common gene mutation tests.
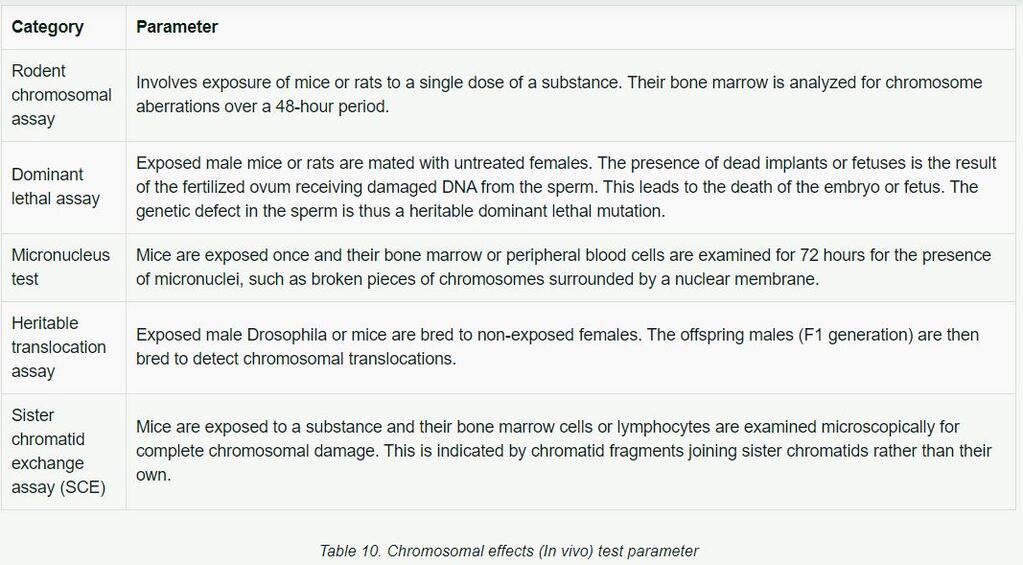
Chromosomal effects can be detected with a variety of tests, some of which utilize entire animals (in vivo) and some which use cell systems (in vitro). Several assays are available to test for chemically induced chromosome aberrations in whole animals. Table 10 lists common in vivo means of testing chromosomal effects.
In Vitro Testing
In vitro tests for chromosomal effects involve exposure of cell cultures and followed by microscopic examination of them for chromosome damage.
The most commonly used cell lines are Chinese Hamster Ovary (CHO) cells and human lymphocyte cells. The CHO cells are easy to culture, grow rapidly, and have a low chromosome number (22), which makes for easier identification of chromosome damage.
Human lymphocytes are more difficult to culture. They are obtained from healthy human donors with known medical histories. The results of these assays are potentially more relevant to determine effects of xenobiotics that induce mutations in humans.
Two widely used genotoxicity tests measure DNA damage and repair that is not mutagenicity. DNA damage is considered the first step in the process of mutagenesis. Common assays for detecting DNA damage include:
- Unscheduled DNA synthesis (UDS) — involves exposure of mammalian cells in culture to a test substance. UDS is measured by the uptake of tritium-labeled thymidine into the DNA of the cells. Rat hepatocytes or human fibroblasts are the mammalian cell lines most commonly used.
- Exposure of repair-deficient E. coli or B. subtilis — DNA damage cannot be repaired so the cells die or their growth may be inhibited.
Emerging Approaches and Methods
In the future, there will likely be additional and refined in vitro methods, and the emergence of in silico and "chip" approaches.
Many current efforts are underway to refine, develop, and validate in vitro methods.
Did you know?
The Human Toxicology Project Consortium provides a video series called Pathways to a Better Future. These videos discuss the future of toxicology, if you would like to know more about where the field is headed.
In Silico Methods
Also emerging are in silico methods, meaning "performed on computer or via computer simulation." This term was developed as an analogy to the Latin phrases in vivo and in vitro.
Advanced computer models called "Virtual Tissue Models" are being developed by the U.S. EPA's National Center for Computational Toxicology (NCCT). The EPA's Virtual Tissue Models are described as using "new computational methods to construct advanced computer models capable of simulating how chemicals may affect human development. Virtual tissue models are some of the most advanced methods being developed today. The models will help reduce dependence on animal study data and provide much faster chemical risk assessments" (source).
One example is the Virtual Embryo (v-Embryo™) research effort, aimed at developing prediction models to increase our understanding of how chemical exposure may affect unborn children. Researchers are integrating new types of in vitro, in vivo, and in silico models that simulate critical steps in fetal development. Virtual Embryo models simulate biological interactions observed during development and predict chemical disruption of key biological events in pathways that is believed to lead to adverse effects.
Figure 4. v-Embryo logo
"Chip" Models
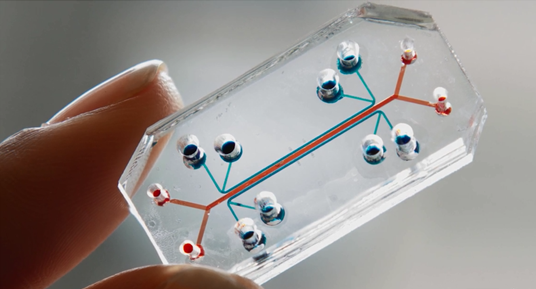
Also emerging are microphysiological systems (MPS) that are used in "tissue chip" and "organs-on-chips" models. Chip models include human cell cultures that are placed on a computer chip and studied there. The Wyss (pronounced "Veese") Institute for Biologically Inspired Engineering is a helpful resource for more information.
For example, the "Lung-on-a-chip" is described as "combining microfabrication techniques with modern tissue engineering, lung-on-a-chip offers a new in vitro approach to drug screening by mimicking the complicated mechanical and biochemical behaviors of a human lung." To learn more, watch a video from the Wyss Institute that shows a human lung-on-a-chip. Another Wyss Institute video illustrates how researches have used long-on-a-chip to mimic pulmonary edema.
Figure 5. Lung-on-a-chip used to mimic pulmonary edema
(Image Source: The Wyss Institute for Biologically Inspired Engineering)
(Image Source: The Wyss Institute for Biologically Inspired Engineering)
Using a connected series of tissue chips as an integrated multi-organ system can allow for the creation of a "human-on-a-chip," to be used to model the metabolism and effects of drugs and other substances moving through a human. For example, a liver chip could provide fluids and metabolites to a kidney chip, allowing for the assessment of the nephrotoxic (kidney damage) potential of a substance metabolized in the liver.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) are an emerging approach using in vitro cultures of cells. The cells of mammals and plants can be reprogrammed via "cellular reprogramming" to generate iPSCs. Like human embryonic stem cells, iPSCs are pluripotent (capable of giving rise to several different cell types) and these cells can renew themselves. As examples, iPSC-derived hepatocytes, cardiomyocytes, and neural cells can serve as tools for the screening of drugs and other substances for potential toxicity, and also can be used to study disease mechanisms and pathways. Further, iPSCs have been studied in immunotherapy and regenerative cellular therapies.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) are an emerging approach using in vitro cultures of cells. The cells of mammals and plants can be reprogrammed via "cellular reprogramming" to generate iPSCs. Like human embryonic stem cells, iPSCs are pluripotent (capable of giving rise to several different cell types) and these cells can renew themselves. As examples, iPSC-derived hepatocytes, cardiomyocytes, and neural cells can serve as tools for the screening of drugs and other substances for potential toxicity, and also can be used to study disease mechanisms and pathways. Further, iPSCs have been studied in immunotherapy and regenerative cellular therapies.
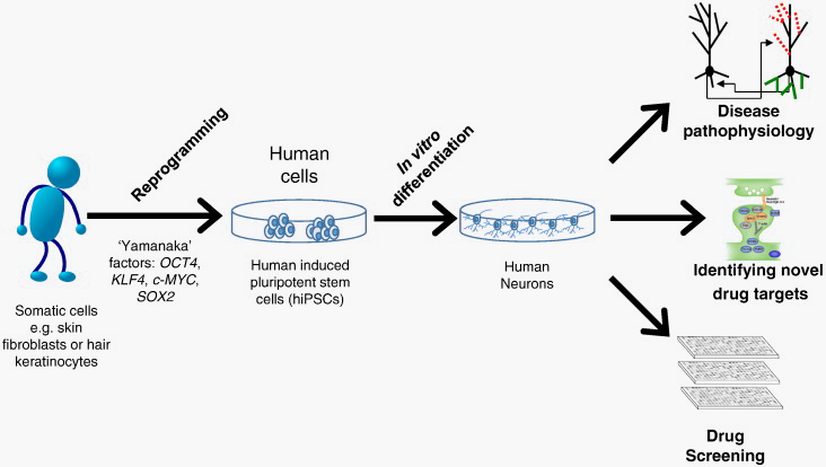
Figure 6. Promise of hiPSCs. Schematic representation of how somatic cells taken from a patient can be reprogrammed into induced pluripotent stem cells (iPSCs) using the ‘Yamanaka’ factors, OCT4, KLF4, c-MYC and SOX2. Subsequent differentiation of human iPSCs (hiPSCs) into neurons of define lineage allow for investigations into disease pathophysiology and identification of potential drug targets. In addition, hiPSC derived neurons may function as a cellular platform in which drug screens can be carried out using disease relevant neurons.
(Image Source: Adapted under Creative Commons Attribution License (CC BY).
doi: 10.1016/j.yhbeh.2015.06.014
Original image: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4579404/figure/f0005/)
(Image Source: Adapted under Creative Commons Attribution License (CC BY).
doi: 10.1016/j.yhbeh.2015.06.014
Original image: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4579404/figure/f0005/)
Did you know?
Professor Shinya Yamanaka (Kyoto University, Japan) received the 2012 Nobel Prize in Physiology or Medicine for discovering that mature cells can be reprogrammed to iPSCs that can differentiate into any type of cell. Key to this discovery was his use of four "reprogramming factors" referred to as c-Myc, Klf4, Oct3/4, and Sox2. Learn more
Combining "Chips" and iPSCs
The emerging approaches of "chips" and iPSCs are being combined. One example is for the evaluation of drugs as potential countermeasures for biological and chemical threats that can be a substitute for human clinical trials. The "chips" and "humans on a chip" can be used as complex in vitro human models to simulate the biology and function of an organ.