Dose Estimates of Toxic Effects
Dose Estimates
Dose-response curves are used to derive dose estimates of chemical substances.
Historically, LD50 (Lethal Dose 50%) has been a common dose estimate for acute toxicity. It is a statistically derived maximum dose at which 50% of the group of organisms (rat, mouse, or other species) would be expected to die. LD50 testing is no longer the recommended method for assessing toxicity because of the ethics of using large numbers of animals, the variability of responses in animals and humans, and the use of mortality as the only endpoint. Regulatory agencies use LD50 only if it is justified by scientific necessity and ethical considerations.
The Three Rs
The current practice for estimating acute toxicity emphasizes the following approaches, known as the Three Rs:
Up-and-down-method - Animals are dosed one at a time. If an animal survives, the dose for the next animal is increased; if it dies, the dose is decreased. The up-and-down procedure would require only 6 to 10 animals, provided that the initial estimate of the LD50 is within a factor of two of the true LD50.
Lethal Doses/Concentrations
Effective Doses (EDs)
Effective Doses (EDs) are used to indicate the effectiveness of a substance. Normally, effective dose refers to a beneficial effect such as relief of pain. It may also stand for a harmful effect such as paralysis. Thus, the specific endpoint must be indicated. The usual terms are:
Dose Estimates
Dose-response curves are used to derive dose estimates of chemical substances.
Historically, LD50 (Lethal Dose 50%) has been a common dose estimate for acute toxicity. It is a statistically derived maximum dose at which 50% of the group of organisms (rat, mouse, or other species) would be expected to die. LD50 testing is no longer the recommended method for assessing toxicity because of the ethics of using large numbers of animals, the variability of responses in animals and humans, and the use of mortality as the only endpoint. Regulatory agencies use LD50 only if it is justified by scientific necessity and ethical considerations.
The Three Rs
The current practice for estimating acute toxicity emphasizes the following approaches, known as the Three Rs:
- Replacing animals in science by in vitro, in silico (performed on computer or via computer simulation), and other approaches.
- Reducing the number of animals used. For example, the oral LD50 approach has been replaced in some circumstances by an up-and-down method (definition below) in which animals are dosed one at a time.
- Refining care and procedures to minimize pain and distress.
Up-and-down-method - Animals are dosed one at a time. If an animal survives, the dose for the next animal is increased; if it dies, the dose is decreased. The up-and-down procedure would require only 6 to 10 animals, provided that the initial estimate of the LD50 is within a factor of two of the true LD50.
Lethal Doses/Concentrations
- Lethal Dose 0% (LD0) — represents the dose at which no individuals are expected to die. This is just below the threshold for lethality.
- Lethal Dose 10% (LD10) — refers to the dose at which 10% of the individuals will die.
- Lethal Concentration 50% (LC50) — for inhalation toxicity, air concentrations are used for exposure values. The LC50 refers to the calculated concentration of a gas lethal to 50% of a group. Occasionally LC0 and LC10 are also used.
Effective Doses (EDs)
Effective Doses (EDs) are used to indicate the effectiveness of a substance. Normally, effective dose refers to a beneficial effect such as relief of pain. It may also stand for a harmful effect such as paralysis. Thus, the specific endpoint must be indicated. The usual terms are:
Toxic Doses (TDs)
Toxic Doses (TDs) are used to indicate doses that cause adverse toxic effects. The usual dose estimates include:
Toxic Doses (TDs) are used to indicate doses that cause adverse toxic effects. The usual dose estimates include:
Determining the Relative Safety of Pharmaceuticals
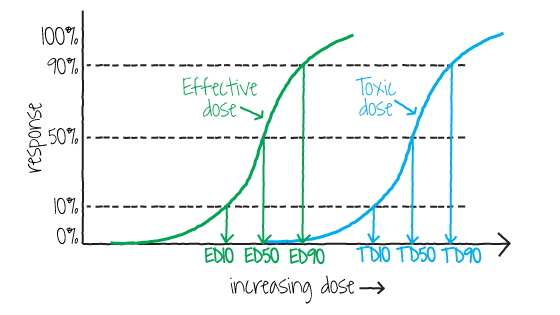
Toxicologists, pharmacologists, and others use effective and toxic dose levels to determine the relative safety of pharmaceuticals. As shown in Figure 1, two dose-response curves are presented for the same drug, one for effectiveness and the other for toxicity. In this case, a dose that is 50% to 75% effective does not cause toxicity. However, a 90% effective dose may result in a small amount of toxicity.
Figure 1. Dose-response curves representing effective dose and toxic dose for the same drug
(Image Source: NLM)
(Image Source: NLM)
It should be noted that a desired effect in a drug is often an undesired effect with an environmental chemical.