Subtopic 3.4: Protein Translation
Protein synthesis requires the interaction of mRNA, tRNA, several accessory proteins, called initiation factor (IF) and elongation (EF) factor, and ribosomes.
|
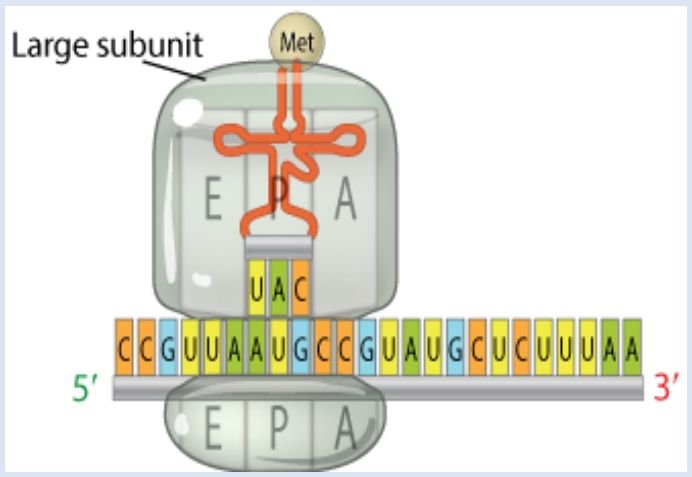
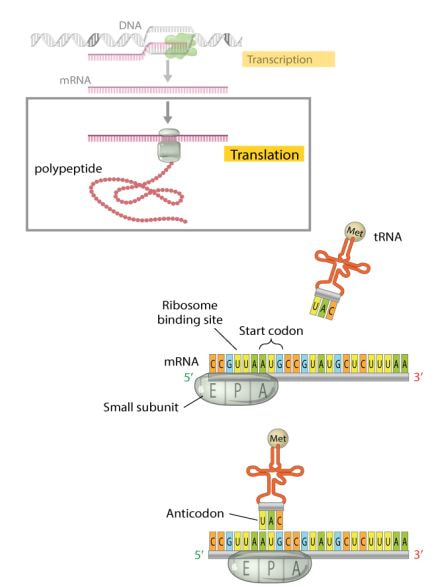
The large ribosomal subunit binds to the small ribosomal subunit to complete the initiation complex. The tRNA molecule bind to one amino acids at the top of the structure. In the base if the tRNA there is the three base sequence known as the anticodon. This anticodon binds, through base pairing, to a three base codon on mRNA. It is this interaction between the mRNA and the amino acid-tRNA that provides the high degree of fidelity observed in the transfer of genetic information from DNA to proteins. |
|
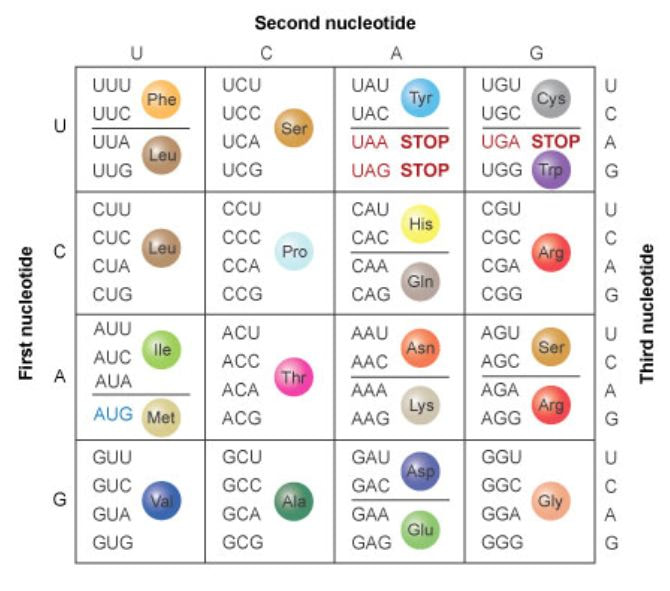
The Triplet Code (Genetic Code) A sequence of three bases in DNA identifies each of the 20 amino acids that are to be incorporated into the newly synthesized protein. This information is incorporated into mRNA which is synthesized using DNA as the template. Considering that three bases are required at a minimum, and we have 4 nucleotides ( 43 = 64 code words are possible). This is more than the 20 amino acids. In fact, many triplets are used to define one same amino acid. In addition, some “extra” triplet sequences are used as stop codons to terminate protein synthesis. AUG is used as the start codon for the N-terminal amino acid in eukaryotes. |
Using the same binding rules as DNA double strands (e.g., a tRNA that binds the starting mRNA codon AUG has an anticodon sequence of UAC), insuring the specific order of AAs required for proper production of the protein.
RNA plays three distinct and important roles:
RNA plays three distinct and important roles:
- mRNA – the intermediary between gene and protein – provides the message.
- tRNA – the key or adaptor – reads the genetic code, brings amino acids to the growing polypeptide chain
- rRNA – in ribosome, provides a scaffold for protein synthesis, catalyzes peptide bond formation
The IF and EF accessory proteins serve a number of roles, including enabling binding of the mRNA molecule to the ribosome, movement of the mRNA along the ribosome to the start point of the synthesis, docking of the tRNA–amino acid, and movement of the mRNA and growing peptide chain, as well as accuracy assurance
The IF and EF accessory proteins serve a number of roles, including enabling binding of the mRNA molecule to the ribosome, movement of the mRNA along the ribosome to the start point of the synthesis, docking of the tRNA–amino acid, and movement of the mRNA and growing peptide chain, as well as accuracy assurance
|
The protein biosynthesis, can be divided into three phases: initiation, elongation, and termination.
|